Unknown Chemical(s) Guidance
An unknown is defined as a chemical in an unlabeled container for which the identity is undetermined. Federal, state, and local regulations all specifically prohibit the transportation, storage, or disposal of unknown wastes. In addition, hazardous waste disposal companies will not accept unknowns without proper analysis. Therefore, unknown or unlabeled chemicals all require analysis prior to disposal, which can easily cost $1,000 or more for a single sample. Unknown chemicals present serious legal and safety problems for the University.
-
Preventing Unknown Chemicals
Many unknown chemicals are generated due to a lack of good housekeeping and good laboratory safety practices. Unknown and unlabeled chemicals can be prevented by:
- Labeling all containers (including beakers and test tubes) properly. This should be done even when creating reagent solutions for temporary use. Labeling will also prevent using the wrong material accidentally.
- Inspecting containers and labels in the lab inventory periodically and replacing fading or deteriorating labels that are not legible.
- Labeling containers using full chemical names, not abbreviations, chemical structures, or formulas.
- Archived research samples are often stored in boxes containing hundreds of small vials. Label the outside of the box with the chemical constituents, paying special attention to regulated materials such as radioactives, solvents, heavy metals, and other toxic materials. If the samples are nonhazardous, label them as such.
- Maintaining an accurate chemical inventory list.
- Require all reaction mixtures stored in lab glassware to be labeled with chemical composition, the date they were formed, and the name of the lab worker responsible.
- Require all workers to properly identify any unknown materials before they leave the area or lab.
- In the event of lab staff turnover or graduation, require all materials be identified and dispose of all materials and samples that are no longer in use or wanted.
- Submit frequent waste pick up request forms to reduce the amount of hazards in your laboratory to reduce risk and create more lab space.
-
Identifying Unknown Materials
Every effort should be made to properly identify an unknown chemical prior to contacting EHS. The following steps should be taken to help identify unknown and unlabeled chemicals:
- Consult with the Principal Investigator (PI) or Lab Manager about the type of work that was being conducted. Eliminating certain chemicals as a possibility helps narrow down the determination and also helps with final waste disposal. This is especially important for materials that may contain mercury, polychlorinated biphenyls (PCBs), polyfluoroalkyl substances (PFAS), dioxins, and furans, as they require special handling for disposal.
- Ask area personnel about the container.
- Contact groups that previously used that area and see if they can provide any information on the identity of the materials.
- Review projects current in process.
-
Disposal of Unknown Materials
If the identity of the material cannot be determined through all of the steps identified above, EHS will remove the material and dispose of it. However, EHS requires three tests be performed and the results recorded on the hazardous waste tag prior to pick up. EHS will not remove any wastes for disposal without a properly filled out hazardous waste tag on the container, or a properly submitted Hazardous Waste Request in the Safety Portal. In addition, based on the number of unknowns in a lab, EHS may charge the generator or department a fee per bottle for the analysis and identification of the waste.
Visually evaluate the material, if the container is swollen, compromised, damaged or compromised, or shows visible evidence of crystal growth within the container, contact EHS immediately.
Unknown Testing Procedures
All tests performed should be conducted in a functioning fume hood. Use as small a sample as reasonably possible while performing the tests. The results of each test should be noted on the hazardous waste tag prior to pick up by EHS.
Test #1 – Water Miscibility/Reactivity
This test is to determine if the unknown material reacts with water, and also whether the material is soluble in water, or whether it sinks or floats when added to water.
-
Liquid Unknown
Add a small amount of unknown liquid to a new test tube. Slowly allow one drop of deionized water to flow down the inside of the test tube into the unknown liquid. Any endo- or exothermic (i.e. heat or cold) reactions are considered positive for water reactivity and the results must be noted on the hazardous waste tag attached to the unknown. Note, if the unknown material exhibits any signs of reacting with water do not complete any further testing and contact EHS for proper disposal via the EHS Safety Portal Hazardous Waste Request.
If unknown liquid does not react to water as identified above, add a small amount of the unknown liquid to a test tube containing deionized water to determine if the material sinks, floats, or is soluble in water.
Examples:
Unknown Material Floats on Water

Unknown Material Sinks in Water (Water Dyed Green)

-
Solid Unknown
Use a spatula to obtain a small amount of the material as possible and place on a watch glass. Place one drop of water onto the side of the watch glass and allow to run down into the unknown solid. Any endo- or exothermic (i.e. heat or cold) reactions are considered positive for water reactivity and the results must be noted on the hazardous waste tag attached to the unknown. Note, if the unknown material exhibits any signs of reacting with water do not complete any further testing and contact EHS for proper disposal via the EHS Safety Portal Hazardous Waste Request.
Example of Water Drop Being Added to Solid Unknown
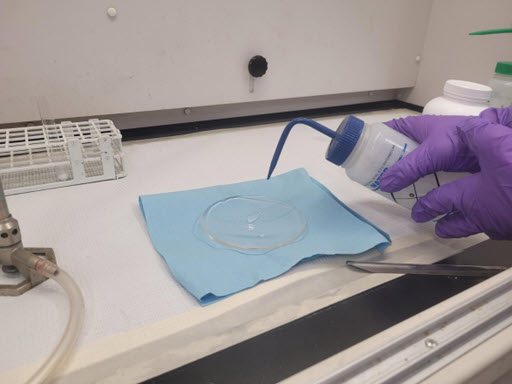
If unknown solid does not react to water as identified above, add an additional amount of the solid to a test tube with deionized water to determine if material is soluble in water. Record results of testing on hazardous waste tag attached to the unknown.
Test #2 – pH
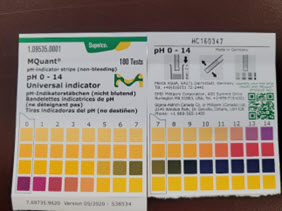
Liquid – Dip the pH paper into the unknown liquid and compare the test strip to the pH chart on the container. Note the results of the test on the hazardous waste tag attached to the unknown.
Example: Unknown liquid has a pH of 0 indicating an acidic solution

Solid – If material from has been determined not to be water reactive, place a small amount of the solid material on a watch glass with enough deionized water to dissolve or wet the solid and dip the pH strip into the solution. Note the results of the test on the hazardous waste tag attached to the unknown.
Example: pH strip being dipped into unknown solid/water solution

Test #3 – Flammability
-
Liquid
Dip cotton swab into unknown liquid and pass-through flame of Bunsen burner. Note results of flammability test as either “positive” or “negative” on hazardous waste tag.
Example: Positive Flammable Reaction

Example: Negative Flammable Reaction

-
Solid
If unknown did not react with water, wet the cotton swab with deionized water and then roll into the solid unknown to coat the swab with the unknown solid material. Pass the cotton swab through the flame of a Bunsen burner. Note the results of the flammability test as either “positive” or “negative” on the hazardous waste tag.

Example: Positive Flammable Reaction

Example: Negative Flammable Reaction


